The discovery of the photoelectric phenomenon dates backs to late eighteenth century AD. The findings related to it have rich historical background with steady progression of understanding of phenomenon. It was in 1905, when Albert Einstein described light as composed of discrete quanta, now called photons. Based upon Max Planck’s theory of black-body radiation, Einstein theorized that the energy in each quantum of light was equal to the frequency multiplied by a constant, later called Planck’s constant. A photon above a threshold frequency has the required energy to eject a single electron, creating the observed effect.
On Photoelectric Effect
The photoelectric effect is the phenomenon that many metals emit electrons when light shines upon them. Electrons emitted in this manner are called photo-electrons. The phenomenon is commonly studied in electronic physics, as well as in field of chemistry, such as quantum chemistry or electro-chemistry.
The detailed explanation is as follows. While electrons are free to move about within a metal, they cannot readily escape it. When high frequency light as ultraviolet or blue light is radiated, electrons pop out of metal with high energy. With lower frequency yellow light, the energy is less. Red light usually emits no electrons. [1]
High frequency light with its high energy photons give electrons enough energy to jump out of metal. As the energy of photons increase, energy of ejected electrons also increases. An increase in the intensity of low-frequency light only increases the number of low-energy photons sent over a given interval of time. This change in intensity will not create any single photon with enough energy to dislodge an electron. Thus, energy of the emitted electrons does not depend on the intensity of the incoming light, but only on the energy (equivalently frequency) of the individual photons. It is an interaction between the incident photon and the outermost electrons.
The lowest frequency of light required to emit electrons from a metal is known as its threshold frequency. For light below this frequency, photons would have insufficient energy to remove an electron from the metal. In case of red light flash, no electrons are ejected.
Electrons absorb energy from photons when irradiated following an “all or nothing” principle. All of the energy from one photon must be absorbed and used to liberate one electron from atomic binding, or else the energy is re-emitted. If the photon energy is absorbed, part of energy liberates the electron from the atom, and the rest contributes to the electron’s kinetic energy as a free particle. [2] [3] [4]
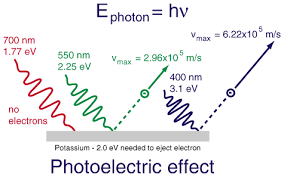
The energy with which the electrons are emitted from a particular metal is measured by the following formula-
Ek = h × (f-f°) (Photoelectric effect formula)
Where Ek = the maximum kinetic energy of the ejected electrons in joules (J).
h = the Plank constant 6.63 x 10-34 J s.
f = frequency of incident photon.
f° = threshold frequency for metal.
And f> f° for photoelectric effect to occur. [5]
Analysis:
We now try and scrutinize the phenomenon from a rational perspective.
Our current scientific understanding of the phenomenon tells us what the occurrence of the phenomenon is and what all is happening.
But we may have never brought upon the following viewpoint as to how would an electron be able to differentiate between incident photons of varying energy (frequency) and respond accordingly? By what method or means it measures the frequency of an incident photon? Decide if it’s above the threshold frequency of the metal, and then escape the metal surface with calculative amount of kinetic energy?
These questions may never have been raised before. But need to be addressed, if we intend to understand the ultimate essential concepts in quantum physics or otherwise.
The discussion leads to an understanding that electrons do comprehend and confirm to the energy photons of different capacity and move out of metal with varying energy correspondingly. It is able to perceive and assimilate its surrounding environment and respond to it accordingly.
So, should it be considered conscious?
Deduction:
The new aspect will help us unveil the disposition of an electron.
The answer to the following question, how does an electron measure the frequency of photon, will help us understand how does it comprehend and confirm to the energy photons of different capacity and move out of metal. And should this “awareness and responsiveness” by electron be compared to what we understand as consciousness?
We will need to defend and justify our reasoning if we stand for or against the argument.
Sources : [1] Bruce Rosenblum, Fred Kuttner (2011).Quantum Enigma: Physics Encounters Consciousness, Oxford University Press. [2] Lenard, P. (1902). "Ueber die lichtelektrische Wirkung". Annalen der Physik. 313 (5): 149–198. doi:1002/andp.19023130510. [3] Millikan, R. (1914). "A Direct Determination of "h."". Physical Review. 4 (1): 73– doi:10.1103/PhysRev.4.73.2. [4] Millikan, R. (1916). "A Direct Photoelectric Determination of Planck's "h""(PDF). Physical Review. 7(3): 355-388. doi:1103/PhysRev.7.355 [5] Fromhold, A. T. (1991). Quantum Mechanics for Applied Physics and Engineering. Courier Dover Publications. pp. 5–6.

